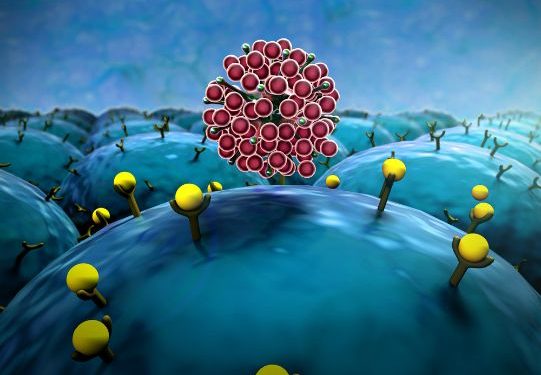
The CSC theory suggests that a subpopulation of stem cells in cancer is responsible for perpetuating it. In 1994, the Dick group studied leukemia-initiating stem cells and confirmed their presence in leukemia-initiating tumors of SCID mice. Subsequent studies showed the presence of stem cells in brain, breast, and other cancers. As a result, the CSC theory has gained considerable acceptance in the field of oncology.
The current model of CSCs has several unique characteristics. In the case of renal cancer, it contains CD105+ CSCs that induce lung metastasis. It also promotes apoptosis in the metastatic stage and induces ERK1/2 signalling. In the case of melanomas, MSCs induce epithelial-mesenchymal transition in the marrow progenitor. Once this process occurs, the remaining MCS migrate short distances before they undergo asymmetric division and disperse throughout the body, resulting in metastasis.
A common hallmark of cancer is deregulation of cellular energetics. CSCs differ from normal cells in their energy metabolism. Metabolic reprogramming may be a key determinant of stemness and resistance mechanisms. In addition to using aerobic glycolysis as their energy source, CSCs switch their energy metabolism to enhanced glycolysis via the upregulation of GLUT1 transporters. The CSCs’ ability to switch to a more aggressive phenotype during cancer treatment is also associated with poorer outcomes in treatment.
Cancer stem cells (CSCs) are mesenchymal cells that are involved in cancer-related fibroblasts. Endothelial cells and cancer-associated fibroblasts, on the other hand, are hematopoietic. These cancer cells can migrate through the bloodstream and elicit a response against a potent chemotherapy drug called temolozomide. In addition, they also have cytokine-mediated mechanisms that promote the formation of new CSC and help tumour survival and recurrence.
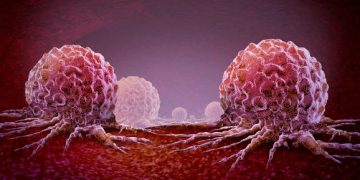
CSCs play crucial roles in tumour progression. They initiate the cancer process and accumulate carcinogenic inducers and oxidative stress. Through mutual interactions with cancer cells, CSCs promote cancer growth and progression by harnessing the tumour microenvironment. Metastatic CSCs are resistant to chemotherapy and have an enhanced ability to spread tumours to distant organs and tissues. Therefore, they are a potential therapeutic target. But there are many challenges associated with the study of CSCs.
Interestingly, inflammatory processes can also trigger the transformation of normal stem cells into cancer stem cells. Furthermore, cancer-related stem cell conversion is preceded by the transition of the stem cell niche. This transition takes place in the presence of high concentrations of LPPs and ROS. Inflammatory cytokines can cause DNA damage in stem cells, which then leads to the initiation of cancer. Cancer stem cells are also genetically altered.
Cancer stem cells are found in mammary gland tumors, where they regulate fibroblasts and microenvironments. Their role in the development of breast cancer is largely unclear, but these cells have been implicated in the regulation of hedgehog signaling. They also play a role in regulating cancer-associated fibroblasts, the precursors of cancer cells. Several studies indicate that cancer stem cells play a critical role in the regulation of tumor growth.